Corporate Overview
If you’re an investor and interested in understanding more about Cymra Life Sciences, please get in touch today: info@cymra.com.au
Newsletter
Strategic Pillars
We have 5 key focus areas which we refer to as our strategic pillars
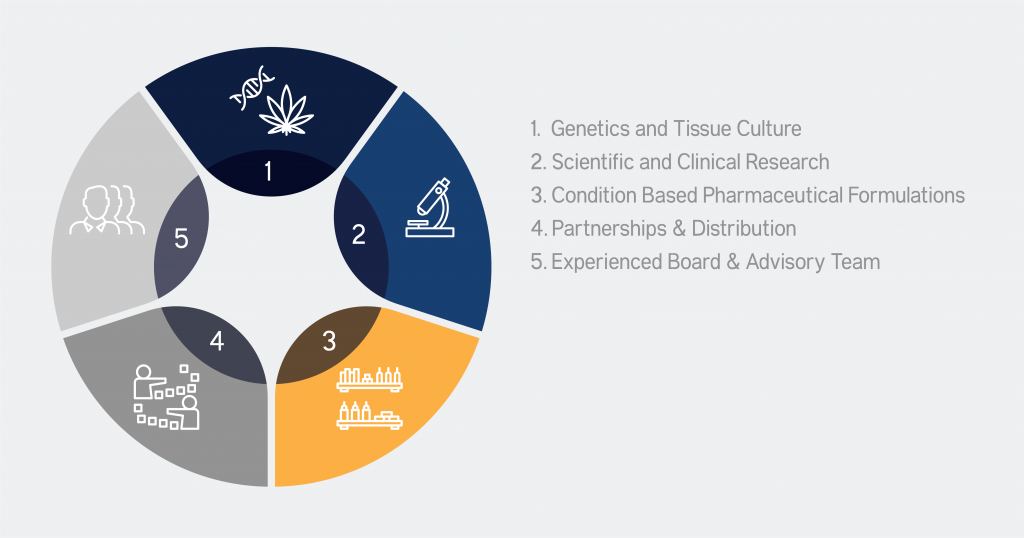
How different are we?
GENETICs
& FLOWER
Locally bred genetics
- Develop and license genetics to medical cannabis cultivators
- Targeted strains
- Pest and Disease resistance
- High yield
Tissue Culture Services
- Provide services to cannabis cultivators for
- Initiation of cultivators of genetics into TC protocols
- Clonal maintenance
One of Australia’s largest cannabis genetic libraries
- Sourced from Germany, The Netherlands, Italy, Spain, Romania, Turkey, Russia and Georgia
- First trial crops underway and well advanced
Commercial Execution
Focus on high value parts of value chain.
- Genetics, high value cannabis extracts – increased margins
Operational Northern Rivers Facility
- Producing GMP grade organically soil grown flower
Distribution Revenue and sales infrastructure
- Existing sales through SAS Cat B
PHARMA DRUG DEVELOPMENT
Clinical Development Plan
- Phase II study completed
- Pre clinical study on cell culture inflammation model for future product releases
Value add by developing proprietary drug
- Chronic pain drug – subject to trial results & TGA
- Clinical claims differentiates + increases margins
Experienced pharma + medical advisory team
- Significant experience in pain + palliative care
- Pharmaceutical experienced board of Directors
If you’re an investor and interested in understanding more about Cymra Life Sciences, please get in touch today: info@cymra.com.au
